Hydrated lime is a critical material for industries such as water treatment, steel manufacturing, sugar processing, construction, and chemical production. Despite its widespread use, the actual manufacturing process inside a lime plant is often misunderstood.
This article explains, in detail, how limestone is transformed into hydrated lime, covering each technical stage from mining and calcination to hydration and quality control while highlighting the industrial importance of process precision and product quality.
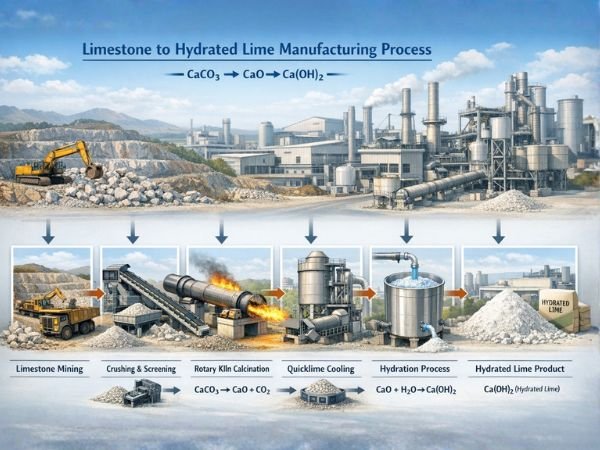
Limestone to Hydrated Lime: Quick Process Summary
- Limestone (calcium carbonate) is mined and crushed
- Crushed limestone is heated in a kiln to produce quicklime
- Carbon dioxide is released during calcination
- Quicklime is cooled and stored safely
- Controlled hydration converts quicklime into hydrated lime
- Final product is used across multiple industrial sectors
This step-by-step transformation defines the limestone to hydrated lime manufacturing process used in modern lime plants.
What Is Limestone and Why Is It Used in Lime Manufacturing?
Limestone is a sedimentary rock primarily composed of calcium carbonate (CaCO₃). Its chemical stability, availability, and predictable reaction under heat make it the ideal raw material for lime production.
Key Properties of Limestone Used in Lime Plants
- High CaCO₃ content
- Low magnesium and silica impurities
- Consistent physical structure
- Suitable size and hardness for kiln processing
The purity of limestone directly affects the reactivity, strength, and quality of the hydrated lime produced.
Step-by-Step Process Inside a Lime Plant
The conversion of limestone into hydrated lime involves controlled thermal decomposition and hydration reactions, carried out using specialized industrial equipment.
Mining and Crushing of Limestone
The process begins at limestone quarries.
Process Overview
- Limestone is extracted through controlled mining methods
- Large stones are transported to crushing units
- Crushers reduce limestone to uniform sizes
Correct sizing is essential to ensure even heat distribution during calcination and consistent lime quality.
Calcination: Converting Limestone into Quicklime
Calcination is the core chemical transformation stage in lime manufacturing.
What Happens During Calcination?
Crushed limestone is heated in kilns at temperatures ranging between 900°C and 1200°C, causing it to decompose.
Chemical Reaction
CaCO₃ → CaO + CO₂
During this reaction:
- Carbon dioxide is released
- Limestone converts into quicklime (calcium oxide)
- The material becomes highly reactive
Kilns Commonly Used
- Rotary kilns
- Vertical shaft kilns
- Regenerative kilns
Precise temperature control is critical to avoid under-calcination or over-burning.
Cooling and Storage of Quicklime
Freshly produced quicklime exits the kiln at extremely high temperatures and must be cooled.
Why Cooling Matters
- Prevents uncontrolled hydration
- Improves handling safety
- Maintains chemical stability
Quicklime is stored in dry, moisture-controlled silos to prevent premature reactions.
Hydration Process: Making Hydrated Lime
Hydration is the controlled addition of water to quicklime to produce hydrated lime (calcium hydroxide).
Hydration Reaction
CaO + H₂O → Ca(OH)₂ + Heat
This is an exothermic reaction, meaning it releases heat.
Key Results of Hydration
- Quicklime expands and breaks down
- Fine, white powder is formed
- Product becomes safer to handle
- Chemical reactivity improves
Careful control of water input ensures consistent particle size and moisture content.
Limestone to Hydrated Lime Process Flow Table
| Stage | Input Material | Process Description | Output |
| Mining & Crushing | Limestone (CaCO₃) | Quarrying and size reduction | Crushed Limestone |
| Calcination | Crushed Limestone | Heating at 900–1200°C | Quicklime (CaO) |
| Cooling | Hot Quicklime | Controlled cooling | Stable Quicklime |
| Hydration | Quicklime + Water | Controlled hydration reaction | Hydrated Lime (Ca(OH)₂) |
| Storage & Packing | Hydrated Lime | Moisture-controlled storage | Finished Product |
Key Machinery Used in a Lime Plant
A modern lime plant uses specialized machinery to maintain efficiency and quality.
Major Equipment Includes
- Limestone crushers
- Rotary or vertical kilns
- Lime coolers
- Hydration systems
- Conveyors and elevators
- Dust collection systems
- Storage silos and packing units
Automation ensures consistent production and minimizes material loss.
Typical Chemical Specifications of Hydrated Lime
| Parameter | Typical Value |
| Ca(OH)₂ Content | 90–95% |
| Moisture Content | Less than 2% |
| Particle Size | 90% passing 325 mesh |
| Reactivity | High |
| Bulk Density | 450–550 kg/m³ |
These specifications are critical for industries requiring precise chemical performance.
Quality Control in Hydrated Lime Manufacturing
Quality control ensures hydrated lime meets industrial and regulatory standards.
Key Quality Checks
- Calcium hydroxide purity
- Particle fineness
- Reactivity rate
- Moisture levels
- Impurity content
Consistent quality improves process efficiency and end-product reliability.
Hydrated Lime Uses by Industry

| Industry | Application |
| Water Treatment | pH control, softening, sludge treatment |
| Steel Industry | Fluxing agent and slag conditioning |
| Sugar Industry | Juice clarification |
| Construction | Mortar, plaster, soil stabilization |
| Chemical Industry | Neutralization and synthesis reactions |
The versatility of hydrated lime makes it indispensable across industries.
Common Mistakes That Affect Hydrated Lime Quality
Even small process errors can significantly reduce lime performance.
Frequent Issues Include
- Using low-purity limestone
- Incomplete or excessive calcination
- Uncontrolled hydration reactions
- Poor storage conditions leading to moisture exposure
Avoiding these mistakes ensures consistent, high-quality hydrated lime.
Environmental and Safety Measures in Lime Plants
Modern lime manufacturing emphasizes sustainability and worker safety.
Environmental Controls
- Advanced dust collection systems
- Emission monitoring
- Efficient fuel utilization
- Waste heat recovery
Safety Practices
- Automated material handling
- Controlled hydration systems
- Moisture-free storage
- Compliance with industrial safety standards
Responsible manufacturing reduces environmental impact while improving efficiency.
Why Manufacturing Expertise Matters in Lime Production
Producing high-quality hydrated lime requires:
- Consistent raw material sourcing
- Process optimization
- Technical knowledge
- Quality-driven operations
Experienced manufacturers understand how each stage affects final product performance and reliability.
Final Thoughts
The transformation from limestone to hydrated lime is a precise, controlled process that demands technical expertise, quality raw materials, and efficient plant operations. Each stage from calcination to hydration directly impacts the purity, reactivity, and performance of the final product used across critical industries.
By following proven manufacturing practices and strict quality control, Shri Jodhpur Lime demonstrates the standards expected from a reliable hydrated lime supplier in India, delivering consistent and application-ready lime products for industrial use
Frequently Asked Questions
Q1. How is limestone converted into hydrated lime?
Answer: Limestone is heated in a kiln to form quicklime, which is then reacted with water in a controlled hydration process to produce hydrated lime.
Q2. What temperature is required for lime calcination?
Answer: Calcination typically occurs between 900°C and 1200°C, depending on kiln design and limestone quality.
Q3. What is the difference between quicklime and hydrated lime?
Answer: Quicklime is calcium oxide and highly reactive, while hydrated lime is calcium hydroxide and safer to handle.
Q4. Where is hydrated lime used?
Answer: Hydrated lime is used in water treatment, steel manufacturing, construction, sugar processing, and chemical industries.
Q5. Is lime manufacturing environmentally safe?
Answer: With modern pollution control systems and efficient kilns, lime manufacturing can be conducted responsibly and sustainably.