CaO Quicklime, also known as Calcium Oxide, is one of the most widely used industrial minerals across multiple sectors, including steel manufacturing, water treatment, construction, and environmental management. Produced through the calcination of high-quality limestone, quicklime plays a critical role in improving industrial efficiency, removing impurities, stabilising soil, and treating wastewater.
Due to its high calcium content, strong alkalinity, and rapid chemical reactivity, CaO quicklime remains an essential raw material for heavy industries. From acting as a flux in steel production to neutralising acidic waste streams, its applications are both diverse and technically significant.
In this article, we explain what CaO quicklime is, how it is manufactured, its chemical and physical properties, and the major industrial applications that make it indispensable in modern industry.
What is CaO Quicklime?
CaO Quicklime is the chemical compound Calcium Oxide. It is a white, highly alkaline, and reactive solid obtained by heating limestone (calcium carbonate – CaCO₃) at high temperatures in a kiln.
Chemical Formula:
CaO
How is Quicklime Produced?
Quicklime is produced through a process called calcination.
When limestone is heated at temperatures above 900°C in a rotary or vertical kiln, it decomposes into calcium oxide and carbon dioxide.
Chemical Reaction:
CaCO3(s)→CaO(s)+CO2(g)CaCO₃ (s) → CaO (s) + CO₂ (g)CaCO3(s)→CaO(s)+CO2(g)
This process removes carbon dioxide and leaves behind highly reactive calcium oxide.
Key Characteristics of CaO Quicklime:
- High calcium purity (typically 85–95% CaO depending on grade)
- Strong alkaline nature
- Highly reactive with water
- Generates significant heat during hydration
- Effective impurity remover in metallurgical processes
Because of its chemical stability in dry conditions and strong reactivity when exposed to moisture, quicklime is carefully handled and stored in controlled environments.
Physical & Chemical Properties of Calcium Oxide
Understanding the properties of CaO quicklime helps explain its wide industrial usage.
Physical Properties
| Property | Description |
| Appearance | White to off-white crystalline solid |
| Odor | Odorless |
| Density | ~3.34 g/cm³ |
| Melting Point | 2,572°C |
| Solubility | Slightly soluble in water (reacts instead of dissolving normally) |
Chemical Properties
- Strong alkaline compound
- Reacts vigorously with water
- Produces calcium hydroxide during hydration
- Absorbs moisture and carbon dioxide from air
- Neutralizes acidic compounds effectively
Reaction with Water (Slaking Process)
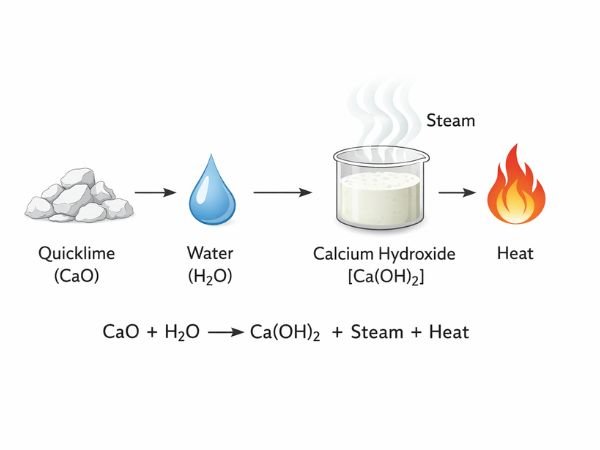
When CaO reacts with water, it forms calcium hydroxide (Ca(OH)₂) and releases heat.
CaO+H2O→Ca(OH)2+HeatCaO + H₂O → Ca(OH)₂ + HeatCaO+H2O→Ca(OH)2+Heat
This reaction is called slaking, and it is an important step in many industrial applications.
The heat generated during hydration makes quicklime useful in processes requiring thermal energy.
Major Uses of CaO Quicklime
CaO quicklime serves as a critical raw material in multiple industries due to its high reactivity and alkalinity.
Quicklime in the Steel Industry
The steel industry is one of the largest consumers of industrial quicklime.
In steel manufacturing, CaO quicklime acts as a fluxing agent. It removes impurities such as:
- Silica
- Phosphorus
- Sulfur
By reacting with these impurities, quicklime forms slag, which can be separated from molten metal. This improves:
- Steel purity
- Structural strength
- Production efficiency
High calcium quicklime is especially preferred in steel plants due to its better reactivity and impurity removal capacity.
Quicklime in Water & Wastewater Treatment
CaO quicklime plays a key role in water purification and wastewater treatment.
Its applications include:
- Neutralizing acidic water
- Removing heavy metals
- Softening hard water
- Controlling pH levels
- Reducing biological contamination
In industrial effluent treatment plants, quicklime helps in precipitation of dissolved impurities, making wastewater safer for discharge or reuse.
Because of its strong alkalinity, it is widely used in municipal water treatment facilities across India.
Quicklime in Construction & Infrastructure
In the construction industry, quicklime is used for:
- Soil stabilization
- Road base improvement
- Improving load-bearing capacity
- Reducing soil plasticity
When mixed with clay soils, CaO reacts with moisture and improves soil strength. This makes it valuable in highway construction and infrastructure development projects.
It is also used in:
- Mortar production
- Cement blends
- Autoclaved aerated concrete blocks
Quicklime in Agriculture & Soil Stabilization
In agriculture, quicklime helps improve soil quality by:
- Reducing soil acidity
- Increasing pH levels
- Enhancing nutrient availability
- Improving crop yield
Acidic soils reduce fertiliser efficiency. By applying lime, farmers can balance soil chemistry and enhance productivity.
Quicklime in Chemical Manufacturing
Calcium oxide is used as a base material in several chemical processes, including:
- Production of calcium carbide
- Manufacturing precipitated calcium carbonate (PCC)
- Glass production
- Sugar refining
Its high temperature stability and reactivity make it a preferred industrial chemical.
Environmental Applications of Quicklime
Environmental management sectors rely on CaO quicklime for:
- Flue gas treatment
- Sulfur removal
- Neutralization of hazardous waste
- Landfill stabilization
Quicklime helps reduce environmental pollution by controlling emissions and treating contaminated materials.
Benefits of Using High-Quality CaO Quicklime
Using premium-grade quicklime offers several industrial advantages:
- Higher impurity removal efficiency
- Better chemical reactivity
- Lower material consumption
- Improved process performance
- Reduced operational cost
- Enhanced environmental compliance
Industries prefer high calcium quicklime because purity directly impacts industrial performance.
Quicklime vs Hydrated Lime: What’s the Difference?
Although both are derived from limestone, quicklime (CaO) and hydrated lime (Ca(OH)₂) differ in composition, reactivity, and industrial applications.
| Parameter | Quicklime (CaO) | Hydrated Lime (Ca(OH)₂) |
| Chemical Formula | CaO | Ca(OH)₂ |
| Production Method | Calcination of limestone | Hydration (slaking) of quicklime |
| Reactivity | Highly reactive | Moderately reactive |
| Heat Generation | Produces heat when mixed with water | No heat generation |
| Handling | Requires controlled storage | Easier and safer to handle |
| Major Uses | Steel, construction, soil stabilization | Water treatment, plaster, mortars |
Key Difference
Quicklime reacts directly with water and generates heat, while hydrated lime is the result of that reaction and is more stable for controlled applications.
Industries choose between the two depending on:
- Required reactivity level
- Moisture conditions
- Application process
- Safety requirements
Why Choosing a Reliable Quicklime Manufacturer in India Matters
The quality of CaO quicklime significantly affects industrial output. Poor-grade lime can reduce efficiency, increase waste, and raise operational costs.
When selecting a quicklime manufacturer in India, consider the following:
High CaO Purity Percentage
Higher calcium oxide content ensures better impurity removal and stronger chemical performance.
Consistent Quality Control
Uniform particle size and controlled calcination improve industrial efficiency.
Advanced Kiln Technology
Modern vertical or rotary kilns ensure:
- Proper limestone calcination
- Minimal unburnt core
- Higher reactivity
Reliable Supply & Logistics
Timely bulk delivery is critical for steel plants, construction projects, and water treatment facilities.
Compliance & Certifications
Check adherence to:
- Bureau of Indian Standards (BIS)
- Environmental norms
- Industrial safety standards
For industries seeking consistent supply of high calcium quicklime, working with an experienced manufacturer like Shri Jodhpur Lime ensures dependable quality, proper grading, and bulk availability across India.
Conclusion
CaO Quicklime (Calcium Oxide) remains one of the most important industrial minerals due to its strong alkalinity, high reactivity, and wide range of applications. From acting as a flux in steel production to improving soil strength and treating wastewater, its role in modern industry is both critical and extensive.
The performance of quicklime in industrial processes depends largely on its purity, calcination quality, and consistency. Selecting a trusted manufacturer ensures optimal efficiency, reduced waste, and better overall output.
For industries looking for reliable and high-quality CaO quicklime supply in India, partnering with an experienced manufacturer ensures consistent quality, bulk availability, and dependable logistics support.
For product enquiries and bulk orders, contact Shri Jodhpur Lime to learn more about industrial-grade quicklime solutions.
Frequently Asked Questions
Q1. What is CaO quicklime used for?
Answer: CaO quicklime is used in steel manufacturing, water treatment, construction, soil stabilisation, and chemical production. It removes impurities, neutralises acidity, and improves material strength in various industrial processes.
Q2. How is quicklime produced?
Answer: Quicklime is produced by heating limestone (CaCO₃) at high temperatures in a kiln. This process, called calcination, releases carbon dioxide and forms calcium oxide (CaO).
Q3. Why is quicklime used in the steel industry?
Answer: In steel plants, quicklime acts as a flux. It reacts with silica, sulfur, and phosphorus to form slag, which separates from molten metal, improving steel purity and strength.
Q4. What is the difference between CaO and Ca(OH)₂?
Answer: CaO is calcium oxide (quicklime), while Ca(OH)₂ is calcium hydroxide (hydrated lime). Hydrated lime is formed when quicklime reacts with water.
Q5. Is quicklime hazardous?
Answer: Quicklime is highly alkaline and reacts with moisture, producing heat. Proper handling, protective equipment, and dry storage are necessary to ensure safety.



